【论著】| HMGA2对软脑膜转移黑色素瘤细胞迁移和增殖的影响
时间:2024-06-09 08:00:25 热度:37.1℃ 作者:网络
[摘要] 背景与目的:软脑膜转移是黑色素瘤中枢神经系统转移的一种形式,高迁移率族蛋白A2(high-mobility group protein A2,HMGA2)已被证实在多种肿瘤的发生、发展过程中起重要作用,但在软脑膜转移黑色素瘤细胞中的生物学作用尚未明确。本研究在构建黑色素瘤中枢神经系统转移小鼠模型的基础上,探讨软脑膜转移黑色素瘤与原发部位以及脑实质转移黑色素瘤在细胞迁移、细胞增殖等方面的差异,并进一步明确差异表达基因HMGA2对软脑膜转移黑色素瘤细胞迁移和增殖的影响。方法:通过慢病毒感染构建稳定表达tdTomato和荧光素酶的B16小鼠黑色素瘤细胞(B16-parental cell,B16-Par)。在此基础上,经过体内转移部位适应性筛选,获得B16特异性脑实质转移细胞(B16-brain metastatic cell,B16-BrM)以及B16特异性软脑膜转移细胞(B16-leptomeningeal metastatic cell,B16-LM)。采用细胞划痕实验以及细胞计数试剂盒-8(cell counting kit-8,CCK-8)检测B16-Par、B16-BrM和B16-LM在迁移和增殖能力上的差异。利用转录组测序技术(RNA sequencing,RNA-seq)分析B16-Par、B16-BrM和B16-LM的差异基因表达,筛选出在B16-LM中特异性上调的HMGA2基因,用实时荧光定量聚合酶链反应(real-time fluorescence quantitative polymerase chain reaction,RTFQ-PCR)和蛋白质印迹法(Western blot)对该结果进行验证。对B16-LM特异性上调基因进行基因本体论(Gene Ontology,GO)分析。使用siRNA干扰HMGA2基因在B16-LM中的表达,通过RTFQ-PCR和Western blot实验对敲低效果进行验证。细胞划痕实验和CCK-8实验检测敲低HMGA2对细胞迁移和增殖的影响。利用基因表达综合数据库(Gene Expression Omnibus,GEO)中GSE174401的数据,验证HMGA2基因在患者软脑膜转移黑色素瘤细胞中表达的特异性。结果:与B16-Par相比,经过脑内环境筛选的肿瘤细胞更易于在中枢神经系统定植。B16-LM具有更强的迁移和增殖能力,且上调表达HMGA2基因。GO分析显示,HMGA2与血管生成、细胞增殖等多个生物学过程相关。敲低HMGA2基因的表达可以抑制B16-LM的迁移和增殖。HMGA2在患者软脑膜转移黑色素瘤细胞中表达上调。结论:软脑膜转移黑色素瘤细胞具有相对独特的细胞生物学行为,其通过上调HMGA2基因的表达来促进细胞迁移和增殖。
[关键词] 黑色素瘤;中枢神经系统转移;软脑膜转移;脑转移;HMGA2
[Abstract]Background and purpose: Leptomeningeal metastasis is a form of central nervous system metastasis of melanoma. High mobility group A2 (HMGA2) has been proven to play an important role in the occurrence and development of various tumors, but its biological functions in leptomeningeal metastatic melanoma cells remain unclear. On the basis of building mouse models of central nervous system metastasis of melanoma, this study investigated the differences in cell migration and cell proliferation among leptomeningeal metastatic melanoma cells, primary site melanoma cells and brain parenchymal metastatic melanoma cells, and further clarified the effects of differentially expressed gene HMGA2 on cell migration and proliferation of leptomeningeal metastatic melanoma cells. Methods: B16 mouse melanoma cells (B16-parental cells, B16-Par) stably expressing tdTomato and luciferase were generated by lentiviral infection. Subsequently, B16 specific brain parenchymal metastatic cells (B16-brain metastatic cells, B16-BrM) and B16 specific leptomeningeal metastatic cells (B16-leptomeningeal metastatic cells, B16-LM) were collected after adaptive screening of metastatic sites in vivo. The differences in migration and proliferation among B16-Par, B16-BrM and B16-LM were assessed by wound healing assay and cell counting kit-8 (CCK-8). RNA sequencing (RNA-seq) was used to analyze differential gene expression in B16-Par, B16-BrM and B16-LM, and HMGA2 gene specifically upregulated in B16-LM was screened out. The results were verified by real-time fluorescence quantitative polymerase chain reaction (RTFQ-PCR) and Western blot. Gene ontology (GO) analysis was performed for genes which were upregulated in B16-LM specifically. siRNA was used to interfere with the expression of HMGA2 gene in B16-LM, and the knock-down effect was verified by RTFQ-PCR and Western blot. The effects of knocking down HMGA2 on cell migration and proliferation were detected by wound healing assay and CCK-8 assay. Using GSE174401 data in Gene Expression Omnibus (GEO), the specificity of HMGA2 gene expression in leptomeningeal metastatic melanoma cells from patients was verified. Results: Compared with Par cells, tumor cells screened by the brain environment were more likely to colonize the central nervous system. B16-LM had stronger migration and proliferation abilities, and upregulated the expression of HMGA2 gene. GO analysis revealed that HMGA2 was associated with many biological processes such as angiogenesis and cell proliferation. When the expression of HMGA2 gene was knocked down, the migration and proliferation of B16-LM could be inhibited. HMGA2 was upregulated in leptomeningeal metastatic melanoma cells from patients. Conclusion: Leptomeningeal metastatic melanoma cells had relatively unique cellular characteristics, which promoted cell migration and proliferation by upregulating HMGA2 gene expression.
[Keywords] Melanoma; Central nervous system metastasis; Leptomeningeal metastasis; Brain metastasis; HMGA2
皮肤黑色素瘤是一种恶性程度极高的肿瘤,起源于表皮基底层的黑色素细胞,通常呈黑色或深棕色[1]。原发性黑色素瘤细胞可以脱离原发组织,并通过血液循环等途径转移到全身各处,易发生脑、肺、肝和骨等部位转移。其中,40%~60%的黑色素瘤患者会累及中枢神经系统[2-3],预后较差[4-5]。脑实质转移和软脑膜转移是黑色素瘤在中枢神经系统中的两种不同的转移形式。与发生在脑实质部位的转移[6]不同,发生软脑膜转移的肿瘤细胞会以游离肿瘤细胞的形式随脑脊液循环或黏附于软脑膜定植生长[7-8]。目前,黑色素瘤细胞软脑膜转移的生物学特性和分子机制研究尚浅,缺乏稳定、有效的研究模型。因此,构建中枢神经系统转移模型,探究软脑膜转移黑色素瘤的特性,对抑制黑色素瘤软脑膜转移,延长患者的生存期具有极其重要的意义[9]。
高迁移率族蛋白A2(high-mobility group protein A2,HMGA2)是一种非组蛋白类染色体蛋白,虽不具有直接的转录激活能力,但可通过结合DNA来调节基因的表达[10]。HMGA2在恶性肿瘤中呈高表达,可通过多种机制促进肿瘤的发生、发展[10-12]。其中,HMGA2可以通过远距离染色质互作促进胶质瘤细胞的入侵,与患者较差的预后相关[13]。此外,HMGA2还会对胶质母细胞瘤的干性、侵袭以及致瘤能力起到一定的促进作用[14]。然而,HMGA2在黑色素瘤软脑膜转移过程中发挥的作用尚不清楚。
本文在新建的黑色素瘤中枢神经系统转移模型的基础上,分析原发部位黑色素瘤细胞、脑转移黑色素瘤细胞、软脑膜转移黑色素瘤细胞之间的差异,进一步探究在软脑膜转移细胞中特异性上调HMGA2基因的表达对肿瘤细胞迁移和增殖的影响,旨在为黑色素瘤软脑膜转移的预后和治疗提供新的线索。
1 材料和方法
1.1 材料、试剂和仪器
小鼠黑色素瘤B16细胞系、HEK-293T细胞系购自中国科学院典型培养物保藏委员会细胞库/中国科学院上海生命科学研究院细胞资源中心。5~6周龄C57小鼠购自江苏集萃药康生物科技股份有限公司,饲养在标准条件下(光照周期12 h/黑暗周期12 h,22 ℃)的SPF级动物屏障中,提供灭菌水,小鼠自由饮食。RPMI-1640培养基、DMEM培养基、Opti-MEM培养基、0.25%胰酶-EDTA溶液购自美国Gibco公司,LipofectamineTM2000转染试剂购自美国Invitrogen公司,胎牛血清(fetal bovine serum,FBS)购自美国Gemini公司,Primocin抗生素购自法国InvivoGen公司,Polybrene购自美国MCE公司,mCherry抗体购自英国Biorbyt公司,HMGA2抗体、β-actin抗体购自美国Proteintech公司,HRP标记二抗购自英国Abcam公司,4’,6-二脒基-2-苯基吲哚(4’,6-diamidino-2-phenylindole,DAPI)购自美国Sigma-Aldrich公司,驴血清购自美国Jackson ImmunoResearch公司,细胞计数试剂盒-8(cell counting kit-8,CCK-8)购自美国APExBIO公司,HMGA2 siRNA购自上海吉玛制药技术有限公司,HMGA2、GAPDH引物购自铂尚生物技术(上海)有限公司,TRIzol试剂购自生工生物工程(上海)有限公司,放射免疫沉淀法(radio immunoprecipitation assay,RIPA)裂解液购自上海碧云天生物技术有限公司,实时荧光定量聚合酶链反应(real-time fluorescence quantitative polymerase chain reaction,RTFQ-PCR)采用的RevertAid First Strand cDNA Synthesis Kit、SYBR GREEN Master Mix均购自美国Thermo Fisher Scientific公司。
1.2 细胞培养
小鼠黑色素瘤B16细胞系培养在含有10%的FBS和0.1 mg/mL Primocin的RPMI-1640完全培养基中,HEK-293T细胞系培养在含有10%的FBS和0.1 mg/mL Primocin的DMEM完全培养基中。培养环境为温度37 ℃,CO2体积分数为5%的细胞培养箱,待细胞培养至80%~90%密度时,进行细胞传代。
1.3 病毒包装和感染
首先构建可以同时表达tdTomato和荧光素酶的质粒,然后将其与包装质粒和包膜质粒共转染至细胞融合度为30%~40%的HEK-293T细胞中,并在8 h后更换培养基。在36和72 h后,分别收集HEK-293T细胞培养上清液并过滤。在黑色素瘤细胞密度为30%时,将病毒上清液与完全培养基以4∶1的比例混合,并加入5 µg/mL的polybrene,充分混匀后替换已有的黑色素瘤细胞上清液,并将其置于无菌培养箱中培养。病毒感染48 h后换液,待细胞长满后传代。对表达tdTomato的细胞进行流式细胞术分选后扩增,获得稳定表达tdTomato和荧光素酶的B16亲代细胞系(B16-parental测量了,B16-Par)。
1.4 黑色素瘤脑实质转移小鼠模型构建
小鼠实验通过复旦大学实验动物伦理审查(202207002S)。将稳定表达tdTomato和荧光素酶的B16细胞悬液吸入1 mL注射器中,注射到麻醉小鼠的左心室中,注射完毕后,退出针头,按压止血1 min。当小鼠脑部有明显的生物发光信号并出现显著的神经症状后,对小鼠实施安乐死,解剖并消化鼠脑,对消化后的细胞悬液进行体外培养扩增,将分选后得到的脑实质转移B16细胞命名为B16-Brain-Inter1细胞。将B16-Brain-Inter1细胞再次注射到小鼠左心室中,重复上述步骤后获得B16-Brain-Inter2细胞,再次重复该步骤,获得具有特异性脑实质转移能力的细胞,即获得B16特异性脑实质转移细胞(B16-brain metastatic cell,B16-BrM)细胞。随着注射细胞的驯化次数的增加,细胞特异性脑实质转移能力增强,注射后小鼠出现脑实质转移信号的时间缩短。将B16-BrM注射到小鼠左心室内,以3 d为时间间隔,持续对小鼠进行生物发光成像检测。注射细胞后6 ~ 14 d,可在脑部发现明显的特异性转移信号,且解剖染色后发现B16-BrM转移到脑实质而不是软脑膜部位,说明黑色素瘤脑实质转移小鼠模型构建成功,且B16-BrM可用来进一步实验。
1.5 黑色素瘤软脑膜转移小鼠模型构建
将稳定表达tdTomato和荧光素酶的B16细胞系鞘内注射至小鼠的小脑延髓池中,对其进行驯化。当小鼠头部以及脊柱部位有明显的生物发光信号并出现显著的神经症状后,对小鼠实施安乐死,并收集小鼠脑脊液中的肿瘤细胞。随后对收集到的肿瘤细胞进行体外培养,获得B16-CSF-Inter1细胞。将该细胞再次重复以上驯化步骤后,进行心脏注射,收集脑脊液中的细胞,即B16特异性软脑膜转移细胞(B16-leptomeningeal metastatic cell,B16-LM)。将B16-LM注射到小鼠左心室内,当小鼠脑部出现生物发光信号,且解剖染色后发现B16-LM 转移到软脑膜而不是脑实质部位,说明黑色素瘤软脑膜转移小鼠模型构建成功,且B16-LM 可用来进一步实验。
1.6 生物发光成像
本研究通过腹腔注射Avertin对造模小鼠进行深度麻醉,随后在其眼眶静脉丛注射100 µL 15 mg/mL的D-luciferin,并对肿瘤灶进行成像。
1.7 转录组测序技术(RNA sequencing,RNA-seq)分析
用TRIzol法从3种细胞中提取总RNA,并除去基因组DNA。对RNA浓度进行测量后,每个样本取0.3 µg用于RNA样品制备和文库构建,并在Illumina NovaSeq 6000测序仪上进行高通量测序。主成分分析(Principle Component Analysis,PCA)是一种让数据降维的算法,点之间的距离即代表着样本之间的相似程度。
1.8 免疫荧光染色
对小鼠进行深度麻醉后,依次用PBS和4%PFA进行心脏灌流。解剖后,取出小鼠脑组织并放入4%PFA中固定。12 h后,将脑组织转移至30%蔗糖溶液中至饱和,然后用最佳切削温度复合物(optimal cutting temperature compound,OCT)包埋剂对其进行包埋,用冷冻切片机对冷冻脑组织进行切片。将切片置于含0.3% Triton X-100的PBS中渗透30 min,然后使用10%驴血清封闭1 h,4 ℃一抗温育过夜。用含吐温-20磷酸缓冲盐溶液(phosphate-buffered saline with Tween-20,PBST)洗涤后,荧光二抗室温温育1 h。用PBST洗涤3次,DAPI染色后封片。用Olympus FV3000共聚焦显微镜对载玻片进行成像。
1.9 蛋白质印迹法(Western blot)检测蛋白表达水平
向细胞中加入适量的RIPA裂解液并置于冰上裂解后,使用细胞刮将细胞刮下并置于离心管中,4 °C、12 000 r/min离心10 min,收集到的上清液即为所提取蛋白质。用二喹啉甲酸(bicinchoninic acid,BCA)蛋白检测试剂盒进行定量。用12%十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecylsulphate polyacrylamide gel electrophoresis,SDS-PAGE)分离20 μg蛋白,蛋白分离后,转移到NC膜上,用脱脂奶粉(5%)室温封闭1 h;加入兔抗小鼠HMGA2抗体(1∶ 2 000稀释)、β-actin抗体(1∶5 000稀释)4 °C温育过夜,漂洗,同源二抗(1∶5 000稀释)室温温育1 h,充分漂洗后进行显色。
1.10 RTFQ-PCR检测RNA表达水平
使用TRIzol试剂从细胞中提取总RNA,然后使用RevertAid First Strand cDNA Synthesis Kit将 RNA反转录为cDNA,反应条件:25 ℃ 5 min;42 ℃ 60 min;70 ℃ 5 min终止反应。使用SYBR G reen Mas ter M ix进行RTFQ-PCR。反应体系:3 μ L稀释好的cDNA模板,上下游引物各1 μL,5 μL SYBR Green Master Mix;HMGA2正向引物序列为5’-GAGCCCTCTCCTAAGAGACCC-3’,反向引物序列为5’-TTGGCCGTTTTTCTCC AATGG-3 ’ ,GAPDH正向引物序列为5’-CATCACTGCCACCCAGAAGACTG-3’,GAPDH反向引物序列为5’-ATGCCAGTGAG CTTCCCGTTCAG-3’。
1.11 细胞转染
将B16-LM均匀铺在6孔板内,过夜培养,待细胞密度达到30%~50%时进行转染。按照LipofectamineTM2000转染试剂说明书的步骤分别转染si-NC和si-HMGA2,24或48 h后收集细胞进行后续实验。
1.12 划痕实验检测细胞迁移
将B16小鼠黑色素瘤亲代细胞(B16-Par)、B16-BrM、B16-LM按照合适的比例接种到6孔板进行培养,用移液器吸嘴在细胞上进行划痕。用PBS清洗两次,加入无血清的RPMI-1640培养基,在0、12和24 h拍照记录。B16-LM接种到6孔板并培养24 h,使用转染试剂进行瞬时转染,一定时间后利用移液器吸嘴进行划痕,PBS清洗2次后,加入无血清的RPMI-1640培养基,在0、12和24 h拍照记录。
1.13 CCK-8法检测细胞增殖
将B16-Par、B16-BrM、B16-LM以每孔2 000个细胞的数目接种到96孔板中。培养24 h后,加入10 μL CCK-8溶液,放置暗处培养2 h,用酶标仪在450 nm波长条件下检测各孔的吸光度(D)值,连续测定3 d。将瞬时转染后的B16-LM接种在96孔板中,每孔2 000个细胞,培养24 h后,加入10 μL CCK-8溶液,放置暗处培养2 h,用酶标仪在450 nm波长条件下检测各孔的D值,连续测定2 d。
1.14 公共数据获取和相关分析
利用已公开基因表达综合数据库(Gene Expression Omnibus,GEO)(GSE174401)[3],分别对不同的部位来源的样本进行质控,并运用t分布-随机邻近嵌入(t-distributed stochastic neighbor embedding,t-SNE)降维[15],将高维数据转化为低维数据,并保持其所携带的信息。对不同的细胞类型进行定义和可视化,并对HMGA2基因的表达情况进行分析。
1.15 统计学处理
多组数据间分析采用单因素方差分析,两组之间数据分析使用t检验,计量资料以x±s表示,P<0.05为差异有统计学意义。使用GraphPad Prism 9.4软件进行统计学处理。差异基因分析结果中P值为显著性检验的P值,计算模型为负二项分布。在基因本体论(Gene Ontology,GO)富集分析中P值为显著性检验的P值,其计算基于超几何分布原理。
2 结 果
2.1 构建不同类型的黑色素瘤中枢神经系统转移模型
为构建黑色素瘤中枢神经系统转移模型,本研究通过慢病毒感染构建稳定表达tdTomato和荧光素酶的B16-Par,随后对B16-Par进行脑部转移部位适应性筛选,最终通过心脏注射的方式筛选出B16-BrM和B16-LM肿瘤细胞(图1A)。将以上3类细胞注射到C57小鼠左心室中,6 d后检测小鼠的生物发光成像情况(图1B)。通过免疫荧光染色对造模小鼠肿瘤转移灶的神经解剖部位进行展示, 结果显示,B16-BrM倾向于转移到脑实质,而B16-LM倾向于转移到软脑膜区域(图1C)。
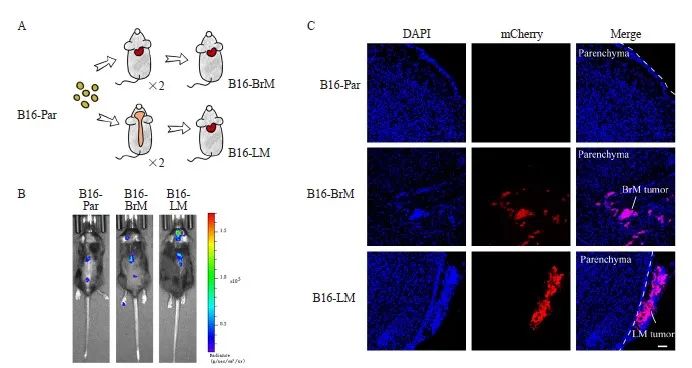
图1 黑色素瘤脑转移与软脑膜转移小鼠模型的构建
Fig. 1 Construction of mouse models of melanoma brain metastasis and leptomeningeal metastasis
A: Screening steps for metastatic cell lines targeting central nervous system; B: Bioluminescence imaging showed that the cell lines passed through the screening for central nervous system metastasis exhibited a stronger ability to metastasize to the central nervous system; C: Immunofluorescence staining revealed that mice injected with B16-BrM cells exhibited brain parenchymal metastasis, while mice injected with B16-LM showed leptomeningeal metastasis. Cancer cells were labeled with mCherry antibody in red, and the nuclei were labeled with DAPI in blue. n=3 per group. Scale bars, 60 μm.
2.2 B16-LM具有更强的迁移和增殖能力
划痕实验结果显示,与B16-Par及B16-BrM相比,B16-LM细胞具有更强的细胞迁移能力,差异有统计学意义(P<0.001,图2A、2B)。CCK-8实验结果显示,B16-LM细胞的增殖能力显著高于B16-Par及B16-BrM,差异有统计学意义(P<0.01,图2C)。
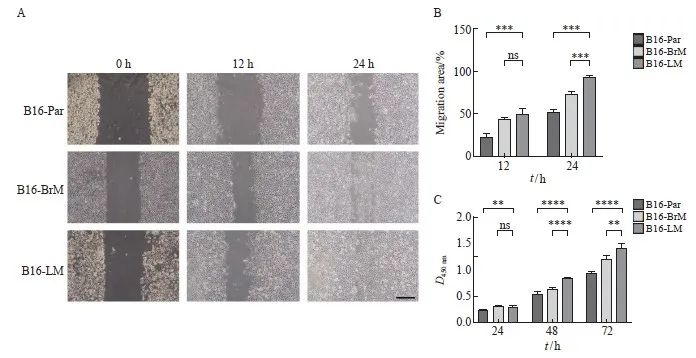
图2 B16-LM细胞具有更强的迁移和增殖能力
Fig. 2 B16-LM cells exhibited enhanced migration and proliferation abilities
A, B: Wound healing assay was performed to assess the migration capacity of B16-Par, B16-BrM and B16-LM. Scale bars, 200 μm; C: CCK-8 assay was conducted to evaluate the proliferation potential of B16-Par, B16-BrM and B16-LM. **: P<0.01, compared with B16-Par or B16-BrM; ***: P<0.001, compared with B16-Par or B16-BrM; ****: P<0.000 1, compared with B16-Par or B16-BrM. ns: No significance.
2.3 B16-Par、B16-BrM和B16-LM各细胞株转录组的主成分分析
为了进一步探究B16-Par、B16-BrM和B16-LM各细胞株之间的差异,本研究通过RNA-seq对这些细胞的转录组进行分析。主成分分析(principal component analysis,PCA)结果显示,B16-Par、B16-BrM和B16-LM的基因表达谱彼此独立(图3),表明B16-LM的转录组与B16-Par及B16-BrM的转录组相比存在一定差异。
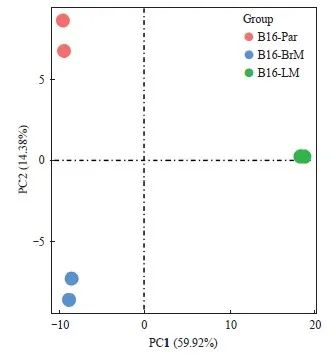
图3 PCA结果显示B16-Par、B16-BrM和B16-LM转录组之间存在差异
Fig. 3 PCA showed distinct transcriptome profiles among B16-Par, B16-BrM and B16-LM
2.4 HMGA2基因在B16-LM中上调表达
本研究对以上3种细胞株进行差异表达基因分析,结果显示,与B16-Par相比,B16-LM中有1 211个基因的表达水平上调(P<0.05,|log2FoldChange|>1,图4A),B16-BrM中有153个基因表达上调(P<0.05,|log2FoldChange|>1,图4B)。对这些上调基因进行韦恩图分析,发现有1 097个基因仅在B16-LM中上调表达(图4C),其中包括HMGA2基因(表1)。此外,本研究还通过RTFQ-PCR和Western blot法,检测HMGA2蛋白在B16-Par、B16-BrM、B16-LM中的表达水平。结果显示,HMGA2在B16-LM中明显高表达(图5)。
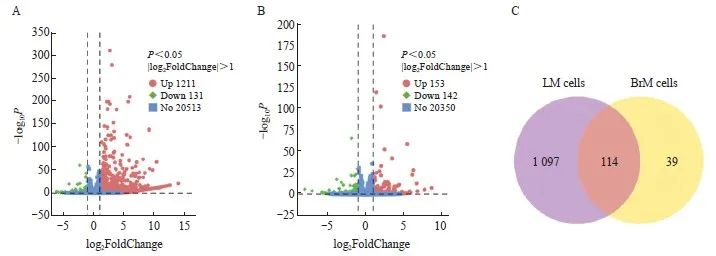
图4 黑色素瘤细胞特性分析
Fig. 4 Characteristics analysis of melanoma cells
A: Differential gene analysis showed that 1 211 genes exhibited increased expression levels in B16-LM compared with B16-Par (P<0.05, |log2FoldChange|>1); B: Differential gene analysis showed that 153 genes in B16-BrM exhibited increased expression levels compared with B16-Par (P<0.05, |log2FoldChange|>1); C: The Venn diagram showed that 1 097 genes were specifically up-regulated in B16-LM.
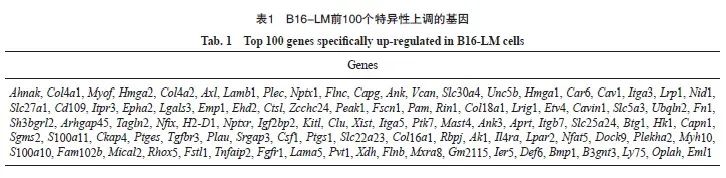
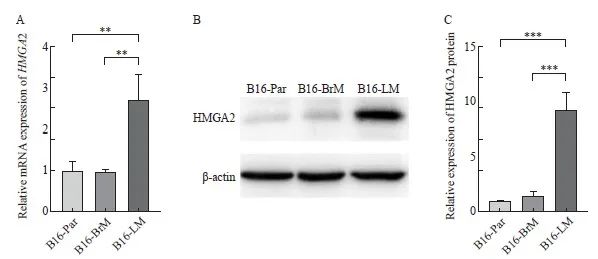
图5 HMGA2基因在B16-LM中上调表达
Fig. 5 The expression level of HMGA2 was up-regulated in B16-LM
A: The mRNA level of HMGA2 gene was detected by RTFQ-PCR; B, C: The expression of HMGA2 protein was measured by Western blot. **: P<0.01, compared with B16-Par or B16-BrM; ***: P<0.001, compared with B16-Par or B16-BrM.
2.5 HMGA2基因与B16-LM中多个生物学过程相关
对B16-LM中特异性上调的1 097个基因进行GO分析,结果显示,多个生物学过程与HMGA2的表达相关,其中包括血管生成、细胞增殖等(P <0.05,图6),表明HMGA2的表达与B16-LM细胞株的迁移和增殖等特性相关。
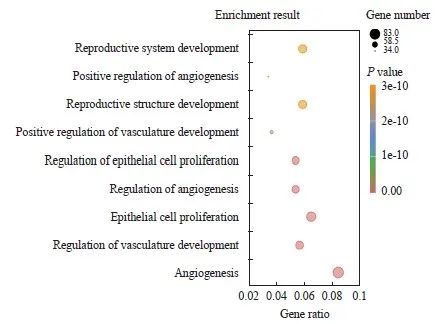
图6 B16-LM中与HMGA2表达相关的上调生物学过程
Fig. 6 Up-regulated biological processes associated with HMGA2 expression in B16-LM
2.6 HMGA2促进B16-LM的迁移和增殖
将沉默HMGA2基因表达的siRNA转染入B16-LM后, 通过RTFQ-PCR和Western blot实验对HMGA2基因表达水平的改变进行验证。结果显示,与对照组相比,HMGA2敲低组的mRNA表达和蛋白水平均明显下降(P<0.01,图7A~C)。细胞划痕实验证明,与对照组相比,敲低HMGA2后的B16-LM的迁移能力受到了抑制,差异有统计学意义(P<0.05,图7D、E)。CCK-8结果表明,si-HMGA2组的细胞生长也受到了抑制,差异有统计学意义(P<0.05,图7F)。
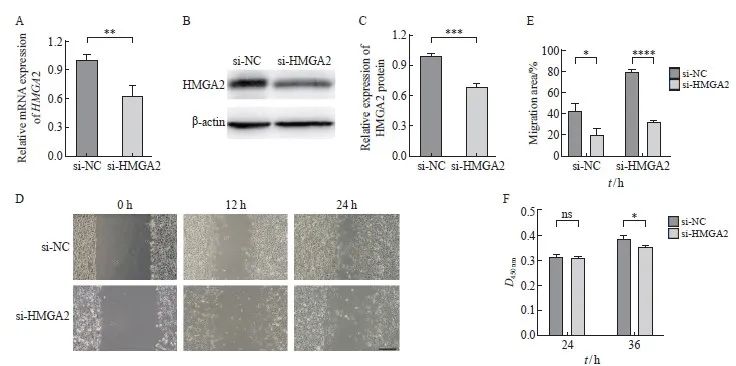
图7 干扰HMGA2表达抑制B16-LM体外迁移和增殖
Fig. 7 Down-regulation of HMGA2 expression inhibited the migration and proliferation abilities of B16-LM in vitro
A: The mRNA level of HMGA2 was detected by RTFQ-PCR after siRNA transfection; B, C: The expression of HMGA2 protein was measured by Western blot after siRNA transfection; D, E: Wound healing assay was conducted to detect the effect of HMGA2 knockdown on the migration ability of B16-LM. Scale bars, 200 μm; F: CCK-8 assay was performed to assess the effect of HMGA2 knockdown on the proliferation potential of B16-LM. *: P<0.05, compared with si-NC; **: P<0.01, compared with si-NC; ***: P<0.001, compared with si-NC; ****: P<0.000 1, compared with si-NC. ns: No significance.
2.7 HMGA2基因在黑色素瘤软脑膜转移患者的肿瘤细胞中上调表达
对GEO(GSE174401)中的8个黑色素瘤皮肤样本、14个脑实质转移样本和19个软脑膜转移样本进行分析。结果显示,原发部位的黑色素瘤和中枢神经系统转移后的黑色素瘤存在不同的微环境(图8A~C)。对表达HMGA2基因的黑色素瘤细胞在不同部位的表达水平进行分析显示,HMGA2在软脑膜转移后的黑色素瘤细胞中表达上调(P<0.000 1,图8D)。这一结果与B16细胞系的实验结果一致,提示该基因可能对软脑膜转移黑色素瘤产生一定的影响。此外,本研究结果还显示,在不同的免疫微环境中均存在一定量的免疫细胞,这可能与HMGA2的表达相关。
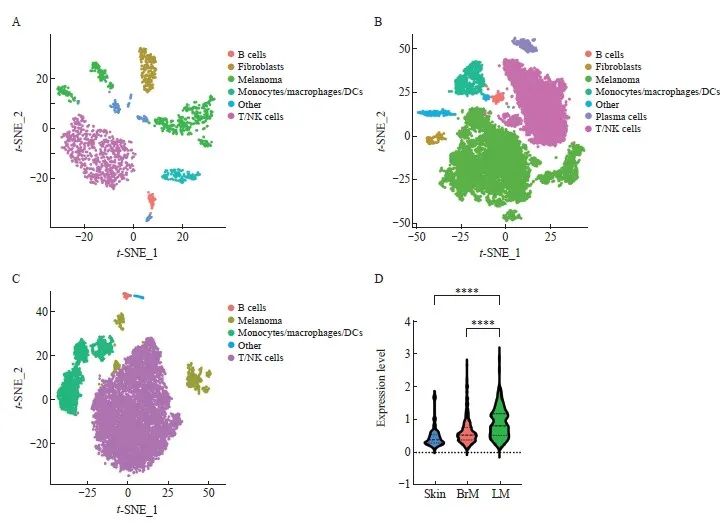
图8 HMGA2在软脑膜转移后的黑色素瘤细胞中表达上调
Fig. 8 The expression level of HMGA2 was up-regulated in melanoma cells after leptomeningeal metastasis
A: t-SNE plot showed the cellular landscape of melanoma in the skin; B: t-SNE plot showed the cellular landscape of melanoma with brain parenchymal metastasis; C: t-SNE plot showed the cellular landscape of melanoma with leptomeningeal metastasis; D: HMGA2 gene expression was up-regulated in melanoma cells after leptomeningeal metastasis. ****: P<0.000 1, compared with skin or BrM. DC: Dendritic cell.
3 讨 论
黑色素瘤是由黑色素细胞恶变形成的肿瘤,常见于皮肤、黏膜等部位[16-17],其恶性程度较高,易发生远端转移[18],是常见的引起软脑膜转移的外周实体瘤之一,晚期患者中的发生率在10%~15%[19],且呈逐年上升趋势[20]。肿瘤软脑膜转移会导致一系列的临床表现,如脑水肿、头痛、恶心等[21],目前仍缺乏安全高效的治疗药物和干预手段[22],患者预后极差。在基础研究中,目前缺乏黑色素瘤软脑膜转移动物模型,相关发生、发展的机制研究尚浅。因此,构建黑色素瘤软脑膜转移模型,深入探究其相关分子机制,对进一步了解软脑膜转移黑色素瘤的特点,以及临床诊断和治疗都具有重要意义。
高迁移率族蛋白(high mobility group protein,HMG)是一组胞内含量丰富的非组蛋白类核蛋白,可分为HMGA、HMGB和HMGN共3个家族。其中,HMGA家族包括两个成员:HMGA1和HMGA2。HMGA2蛋白由HMGA2基因编码,在多种肿瘤细胞中表达,并通过多种机制来促进肿瘤的发生、发展[10]。然而,HMGA2与软脑膜转移黑色素瘤生物学行为之间的联系尚不明确。
因此,本文在构建两种B16黑色素瘤中枢神经系统转移小鼠模型的基础上,对B16-Par、B16-BrM和B16-LM的迁移和增殖能力进行了比较。通过RNA-seq对以上3种细胞株的转录组进行分析,找到了差异表达基因HMGA2。发现敲低B16-LM中HMGA2的表达后,B16-LM的迁移和增殖能力明显下降。在此基础上,利用GEO对原位黑色素瘤患者以及中枢神经系统转移患者的单细胞测序结果进行分析,验证了HMGA2在软脑膜转移后的黑色素瘤中特异性上调表达的 现象。
相关研究[12,23]报道,HMGA2可以通过Hippo-YAP、Wnt/β-catenin等多条信号转导通路促进肿瘤细胞迁移,还可以通过PI3K/AKT/mTOR/p70S6K、cyclin D1/CDK4/CDK6/pRB-E2F1等调控细胞周期,进而影响细胞增殖[10]。而在软脑膜转移黑色素瘤中,HMGA2对其迁移和增殖的调控机制研究仍是空白。此外,在肺癌中,基于紫杉醇的化疗会通过上调HMGA2,刺激CXCL10/CXCL11分泌,增强CD8+T淋巴细胞的浸润[24]。结直肠癌细胞中的HMGA2可以与STAT3启动子结合,激活其转录,诱导CC族趋化因子配体2(CC chemokine ligand 2,CCL2)即MCP-1的分泌,从而促进巨噬细胞招募[25],表明肿瘤中HMGA2的表达确实会对免疫细胞的浸润产生影响。后续我们将进一步探究在软脑膜转移黑色素瘤中HMGA2调控细胞迁移和增殖的具体机制及其与免疫细胞浸润的相关性。
综上所述,HMGA2基因在B16-LM中表达上调,与细胞迁移和增殖等生物学过程相关,敲低HMGA2的表达会抑制B16-LM迁移和增殖,这将为软脑膜转移黑色素瘤的治疗和研究提供新的思路和借鉴。
利益冲突声明:所有作者均声明不存在利益冲突。
作者贡献声明:
李晓辉:设计思路,设计实验方案,实验操作,实验数据分析。
赵加旭:研究方案可行性分析,审核论文。
彭海豹:审核论文。
张 叶:数据分析。
曾 睿:数据分析。
迟喻丹:确定研究对象范围、设计研究方案、设计论文框架、审核修改论文。
[参考文献]
[1]SCHADENDORF D, VAN AKKOOI A C J, BERKING C, et al. Melanoma[J]. Lancet, 2018, 392(10151): 971-984.
[2]KENCHAPPA R S, TRAN N, RAO N G, et al. Novel treatments for melanoma brain metastases[J]. Cancer Control, 2013, 20(4): 298-306.
[3]SMALLEY I, CHEN Z H, PHADKE M, et al. Single-cell characterization of the immune microenvironment of melanoma brain and leptomeningeal metastases[J]. Clin Cancer Res, 2021, 27(14): 4109-4125.
[4]FIFE K M, COLMAN M H, STEVENS G N, et al. Determinants of outcome in melanoma patients with cerebral metastases[J]. J Clin Oncol, 2004, 22(7): 1293-1300.
[5]SAMPSON J H, CARTER J H, FRIEDMAN A H, et al. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma[J]. J Neurosurg, 1998, 88(1): 11-20.
[6]SONG Q Q, RUIZ J, XING F, et al. Single-cell sequencing reveals the landscape of the human brain metastatic microenvironment[J]. Commun Biol, 2023, 6(1): 760.
[7]CHI Y D, REMSIK J, KISELIOVAS V, et al. Cancer cells deploy lipocalin-2 to collect limiting iron in leptomeningeal metastasis[J]. Science, 2020, 369(6501): 276-282.
[8]BOIRE A, ZOU Y L, SHIEH J, et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis[J]. Cell, 2017, 168(6): 1101-1113.e13.
[9]NOLAN C, DEANGELIS L M. Overview of metastatic disease of the central nervous system[J]. Handb Clin Neurol, 2018, 149: 3-23.
[10]MANSOORI B, MOHAMMADI A, DITZEL H J, et al. HMGA2 as a critical regulator in cancer development[J]. Genes (Basel), 2021, 12(2): 269.
[11] HASHEMI M, RASHIDI M, HUSHMANDI K, et al. HMGA2 regulation by miRNAs in cancer: affecting cancer hallmarks and therapy response[J]. Pharmacol Res, 2023, 190: 106732.
[12] XU J X, FANG X J, LONG L Y, et al. HMGA2 promotes breast cancer metastasis by modulating Hippo-YAP signaling pathway[J]. Cancer Biol Ther, 2021, 22(1): 5-11.
[13] ZHANG S, ZHANG H, YU L. HMGA2 promotes glioma invasion and poor prognosis via a long-range chromatin interaction[J]. Cancer Med, 2018, 7(7): 3226-3239.
[14] KAUR H, ALI S Z, HUEY L, et al. The transcriptional modulator HMGA2 promotes stemness and tumorigenicity in glioblastoma[J]. Cancer Lett, 2016, 377(1): 55-64.
[15] VAN DER MAATEN L, HINTON G. Visualizing data using t-SNE [J]. J Mach Learn Res, 2008, 9: 2579-605.
[16] KOZOVSKA Z, GABRISOVA V, KUCEROVA L. Malignant melanoma: diagnosis, treatment and cancer stem cells[J]. Neoplasma, 2016, 63(4): 510-517.
[17] YDE S S, SJOEGREN P, HEJE M, et al. Mucosal melanoma: a literature review[J]. Curr Oncol Rep, 2018, 20(3): 1-10.
[18] 韩如雪, 梁 翔, 马旭辉, 等. 口腔黏膜恶性黑色素瘤肺转移特征及预后分析[J]. 中国癌症杂志, 2022, 32(12): 1184-1189.
HAN R X, LIANG X, MA X H, et al. Characteristics and prognostic analysis of oral mucosal melanoma with lung metastasis[J]. China Oncol, 2022, 32(12): 1184-1189.
[19] STEININGER J, GELLRICH F F, ENGELLANDT K, et al. Leptomeningeal metastases in melanoma patients: an update on and future perspectives for diagnosis and treatment[J]. Int J Mol Sci, 2023, 24(14): 11443.
[20] PAPE E, DESMEDT E, ZAIRI F, et al. Leptomeningeal metastasis in melanoma: a prospective clinical study of nine patients[J]. In Vivo, 2012, 26(6): 1079-1086.
[21] THAKKAR J P, KUMTHEKAR P, DIXIT K S, et al. Leptomeningeal metastasis from solid tumors[J]. J Neurol Sci, 2020, 411: 116706.
[22] RAIZER J J, HWU W J, PANAGEAS K S, et al. Brain and leptomeningeal metastases from cutaneous melanoma: survival outcomes based on clinical features[J]. Neuro Oncol, 2008, 10(2): 199-207.
[23] XIONG L, TANG Y, TANG J, et al. Downregulation of lncRNA HOTTIP suppresses the proliferation, migration, and invasion of oral tongue squamous cell carcinoma by regulation of HMGA2- mediated Wnt/β-catenin pathway[J]. Cancer Biother Radiopharm, 2020, 35(9): 720-730.
[24] LIU C M, ZHENG S F, WANG Z Y, et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer[J]. Cancer Commun (Lond), 2022, 42(9): 828-847.
[25] WANG X, WANG J, ZHAO J H, et al. HMGA2 facilitates colorectal cancer progression via STAT3-mediated tumorassociated macrophage recruitment[J]. Theranostics, 2022, 12(2): 963-975.


